Atrial tachycardia · AT
Reentry is an underlying mechanism in many cases of atrial tachycardia (AT). While the prevalent belief is that AT is mostly driven by a single reentry loop, we hypothesize that this is usually not the case. The key factor behind this lies in the index theorem, prompting a deeper exploration into the atrial topology for a more nuanced understanding.
Anatomy and Bounadries: topology of the left and right atrium
Considering the atria as closed surfaces with holes or boundaries, we can, topologically speaking, conceptualize (deform) the LA and RA as spheres with boundaries. The anatomy of the LA and RA reveals at least three natural openings. For the LA, these include the mitral valve (MV), the left pulmonary veins (LPV), and the right pulmonary veins (RPV). Correspondingly, the RA features openings such as the tricuspid valve (TV), the inferior vena cava (IVC), and the superior vena cava (SVC). Additionally, patients may have non-conductive scar tissue, which, if not connected to natural openings, behaves as additional boundaries, altering the atrial topology. Consequently, we can uniquely identify a patient's atrial topology by counting the number of boundaries in the atria.
It is crucial to note that ablation lines or natural lines of block connecting two boundaries effectively reduce them to a single topological boundary.
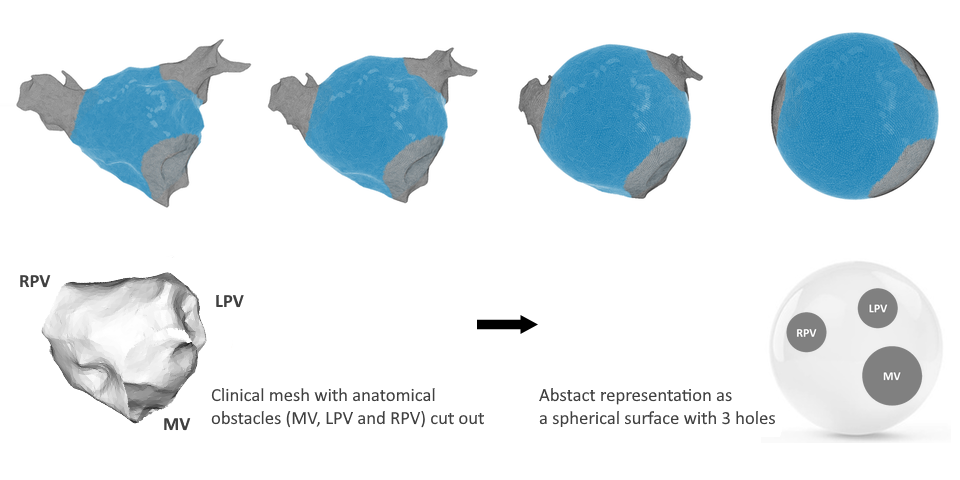 Transformation of left atrium to sphere
Transformation of left atrium to sphereIndex theorem
The index theorem states that the sum of topological charges of all loops on a closed surface (like a sphere) with a finite number of boundaries should be zero (Davidsen et al, 2004) . This has profound implications for AT, as the index theorem suggests that reentrant loops around boundaries will always come in pairs and the number of relevant reentrant loops, that need to be identified for correct treatment, will dependent on the topology of the respective chamber.
In a 2-boundary model, paired rotation essentially mirrors the clockwise and counterclockwise projection of a single reentrant circuit. In the 3-boundary model, paired rotation indicates two clinically relevant loops around two boundaries (index +1, -1) with fused activation (index 0) encircling the third boundary. This principle extends similarly to models with more than three boundaries in the atria.
Complete and incomplete loops
Although one reentry loop will always be obvious as it makes a full rotation (a true loop), the second reentry loop might be suppressed or incomplete due to a collision with the complete rotation, and therefore will not be able to make a clear full rotation. However, calculating the index around a boundary with a suppressed loop will result a ± 1. Therefore, this reentry is equally relevant as a complete loop. We have performed over 1000 computer simulations in 2-boundary, 3-boundary and 4-boundary models which all confirmed our hypothesis.
Ablation
The direct termination of tachycardia can only be achieved by ablating the two boundaries with + and – indices. Failure to ablate the incomplete loop will result in the emergence of a slower AT. This explains why slower ATs often emerge after the first ablation line.
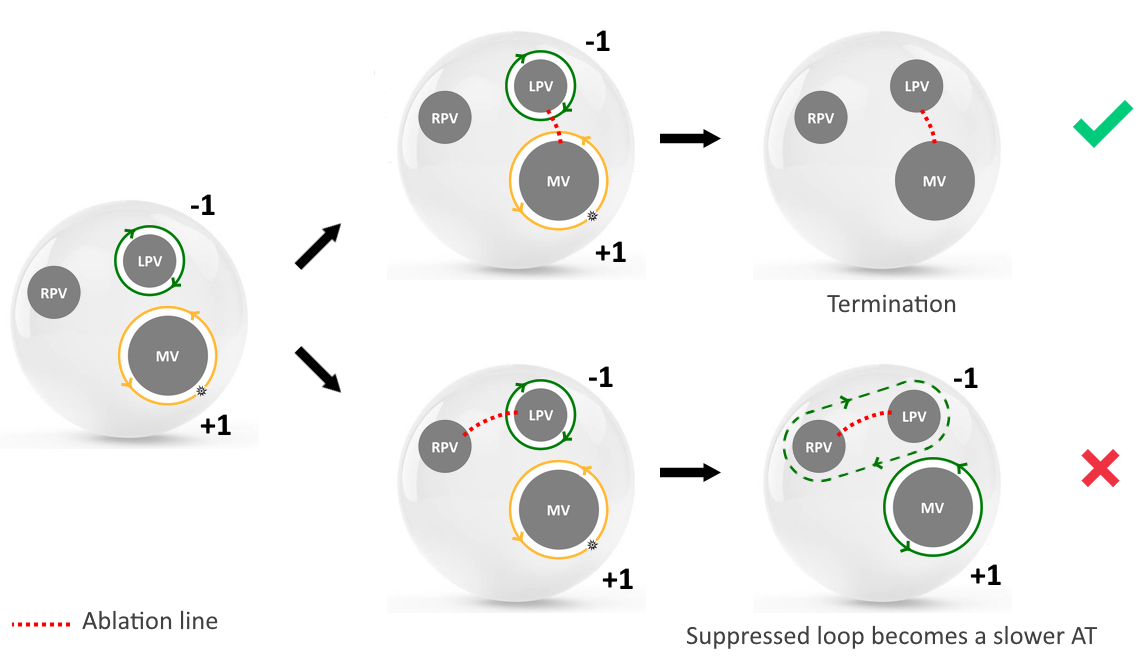 Ablation strategy
Ablation strategyUnique classification of AT
From the index theorem follows that we can uniquely categorize an AT by identifying the number of boundaries and calculating the index of the boundary.
DGM-TOP
We expanded the capabilities of DGM from identifying only complete loops to the enhanced version, DGM-TOP. Unlike its predecessor, DGM-TOP can identify both complete and incomplete loops. To operate, DGM-TOP requires input data from CARTO, RHYTHMIA, or openCARP simulation files, complete with cut-outs of the boundaries. DGM-TOP then accurately identifies the index of each boundary and recommends the optimal ablation line for effective intervention.
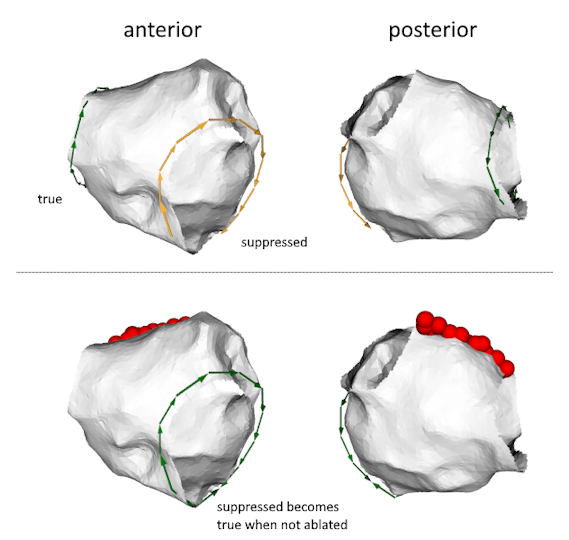 True and Suppressed reentry
True and Suppressed reentryBenefits for the patient
We showed for the first time that suppressed loops play a pivotal role as not ablating them merely leads to slower AT. Due to a full understanding of the mechanism of AT from the first map benefits include operator-independent analysis, a reduction in the need for redo maps, and consequently, shorter procedure times.
Clinical proof
Our work on AT is performed in collaboration with Prof. Mattias Duytschaever and Prof. Sebastian Knecht from the hospital AZ Sint-Jan, Bruges, Belgium, who work with the CARTO system (Biosense Webster) and Dr. Armin Luik and Eng. Annika Haas from the Städtisches Klinikum Karlsruhe, who mainly work with RHYTHMIA (Boston Scientific). We have analyzed over 131 AT cases, which all support our hypothesis.
Further reading
This work was published in European Heart Journal9, while a more detailed version has just been submitted. In previous work, we analyzed the true loops of complex ATs 2 3. Also, an editorial comment (John M. Miller and Tanyanan Tanawuttiwat, JACC EP) was written on the possible promising new apporach of DGM.
Ventricular tachycardia · VT
We have tested DGM on mapped VTs using CARTO (Biosense Webster). Given the similarity in algorithms applicable to both ATs and VTs, our findings reveal that DGM is a rapid, automated algorithm exhibiting a strong agreement with traditional mapping for manually re-annotated VT maps. This collaborative research involved Associate Professor and Electrophysiologist Geoff Lee, along with Dr. and Electrophysiology Fellow Josh Hawson from the Royal Melbourne Hospital. The results of this study, utilizing DGM and thus focusing only on true reentry loops, have been published in JACC: Clinical Electrophysiology6.
Torsade de Pointes · TdP
The initial application of DGM (primitive version) focused on analyzing intramural needle data from the CAVB dog model developed in Prof. M. Vos's lab at Utrecht Medical Center. The primary question was whether TdP is perpetuated by focal activity or reentry. Preliminary analysis using an early version of DGM revealed that short TdP episodes are sustained by focal activity, while longer episodes involve reentry. Non-terminating TdPs consistently showed reentry. The findings of this research have been published in JACC: Clinical Electrophysiology.1.
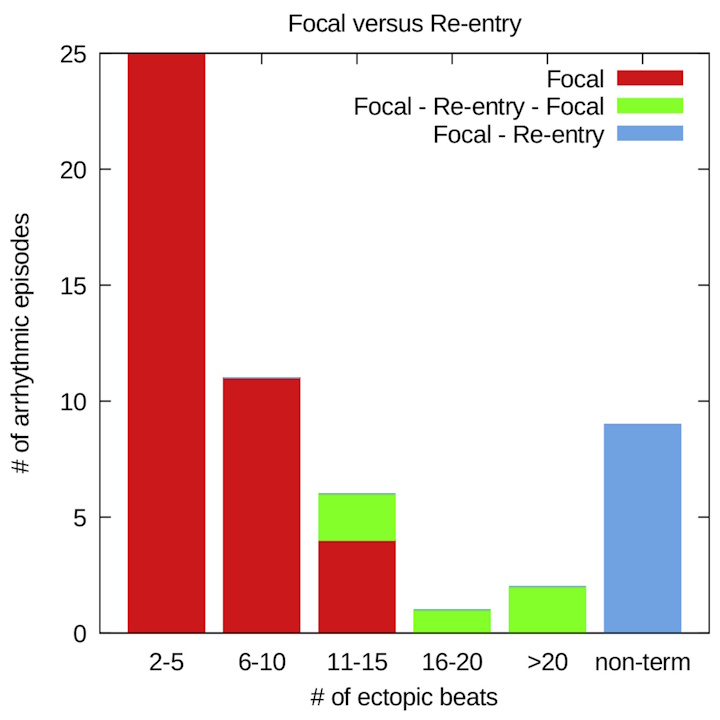
After the results were published, an editoral was written on our work by Sachin Nayyar, Andreu Porta-Sánchez, and Kumaraswamy Nanthakumar (JACC EP). The question was raised how the reentry exactly manifests itself. Is it functional or anatomical? Are there multiple reentries? Is there meandering of the spiral core. This editorial inspired a follow-up paper where we sought to address some of these questions.
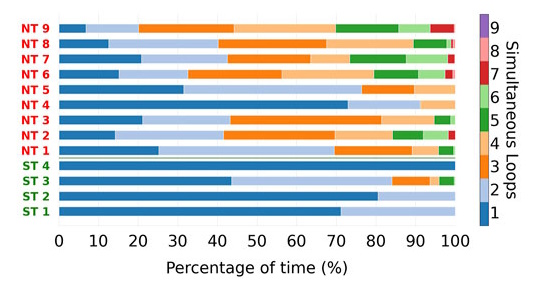
In our follow-up study7, we used the more mature DGM software, which confirmed our previous results. In addition, we found that non-terminating (NT) Torsade de Pointes (TdP) episodes consistently exhibited more simultaneous reentry loops compared to self-terminating (ST) episodes. (Bi-)ventricular loops were prevalent in non-terminating episodes. Thus we found that macro-reentry and multiple simultaneous localized reentries play a role in prolonged TdP. We also found that focal sources (which initiate each episode of TdP) tended to occur in preferred locations, suggesting potential implications for targeted treatment.
Atrial fibrillation · AF
We analyzed a simulated basket catheter data of a meandering spiral wave in the right atrium. When this data was analyzed with phase mapping, many false rotors appeared due to interpolation and far field effects (Martinez-Mateu et al., PLoS Comput Biol, 2018). By changing the parameters of DGM, we were able to differentiate the false rotors from the true rotors. Our manuscript on this study is published in Computers in Biology and Medicine4. Although a meandering spiral is not representative of AF, this was our first step in the direction of non-regular data.
We are currently analyzing simulated datasets of AF. To better understand AF is one of our main goals in the future.
Ventricular fibrillation · VF
We are currently testing DGM and comparing DGM with phase mapping on optical mapping datasets of VF in rats in collaboration with Prof. Dr. Fu Siong (Imperial College London).